The Power Of Structure: A Deep Dive Into The World Of Organic Chemistry
The Power of Structure: A Deep Dive into the World of Organic Chemistry
Related Articles: The Power of Structure: A Deep Dive into the World of Organic Chemistry
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to The Power of Structure: A Deep Dive into the World of Organic Chemistry. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: The Power of Structure: A Deep Dive into the World of Organic Chemistry
- 2 Introduction
- 3 The Power of Structure: A Deep Dive into the World of Organic Chemistry
- 3.1 The Foundation of Structure: Atoms and Bonds
- 3.2 Structure and Reactivity: A Dynamic Duo
- 3.3 Structure and Functionality: A Symphony of Properties
- 3.4 The Importance of Structure: A Catalyst for Progress
- 3.5 FAQs
- 3.6 Tips for Studying Organic Chemistry
- 3.7 Conclusion
- 4 Closure
The Power of Structure: A Deep Dive into the World of Organic Chemistry
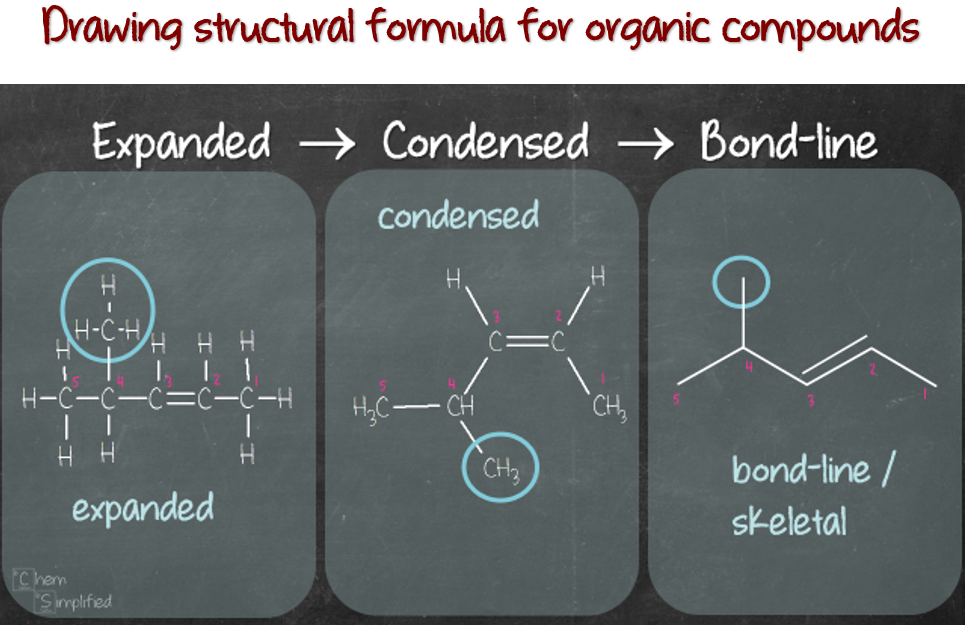
Organic chemistry, the study of carbon-containing compounds, is a vast and intricate field. It underpins countless aspects of our lives, from the food we eat to the medicines we take, and even the materials we use to build our homes. Within this discipline, a core concept that governs the behavior and properties of molecules is their structure. This article delves into the significance of structure in organic chemistry, exploring its role in determining reactivity, functionality, and ultimately, the diverse properties of organic compounds.
The Foundation of Structure: Atoms and Bonds
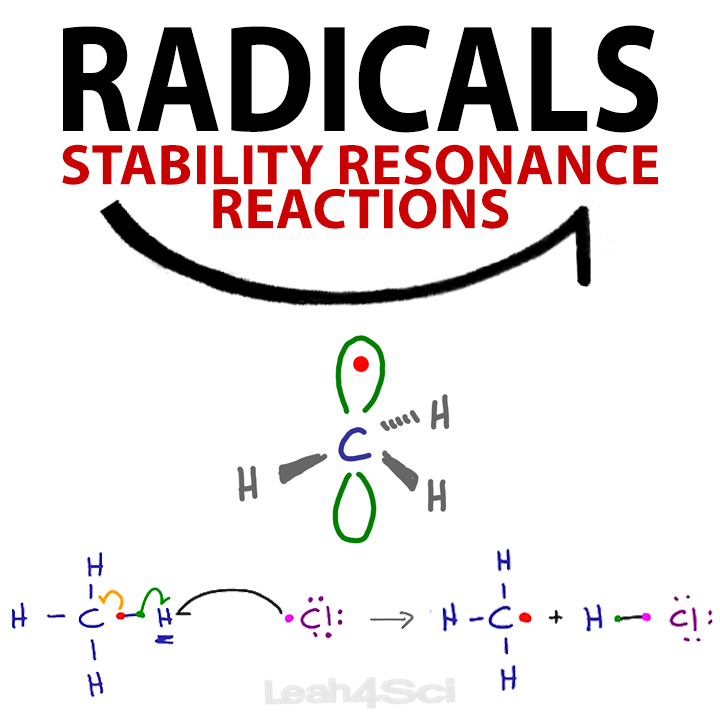
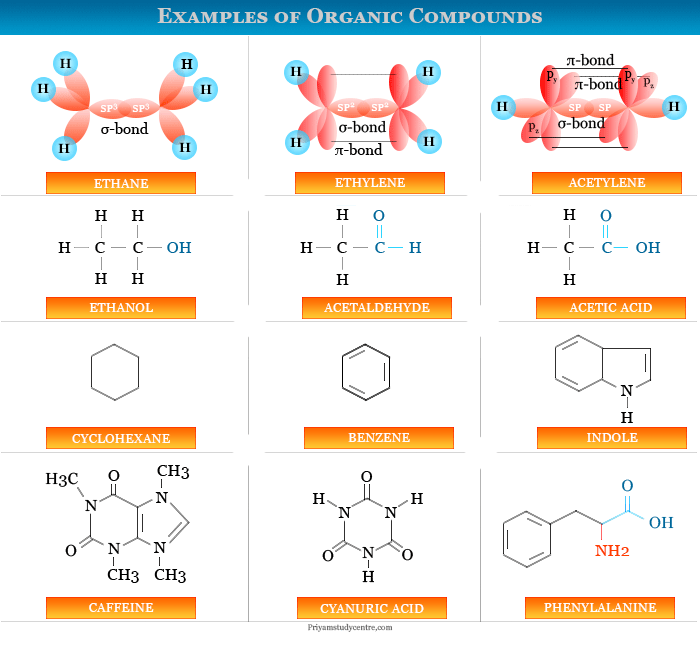
At the heart of organic chemistry lie atoms, the fundamental building blocks of matter. Carbon, the central atom in organic molecules, possesses a unique ability to form strong covalent bonds with itself and other elements, primarily hydrogen, oxygen, nitrogen, and halogens. These bonds, formed by the sharing of electrons, define the arrangement of atoms within a molecule, giving rise to its structure.
The types of bonds formed, their lengths, and the angles between them all play crucial roles in determining the molecule’s shape and its chemical behavior. For instance, the presence of a double bond between two carbon atoms introduces rigidity, influencing the molecule’s overall conformation. Similarly, the presence of polar bonds, where electrons are shared unevenly, can create regions of partial positive and negative charge within the molecule, impacting its interactions with other molecules.
Structure and Reactivity: A Dynamic Duo
The structure of a molecule dictates its reactivity, determining how readily it will undergo chemical reactions. Functional groups, specific arrangements of atoms within a molecule, act as "hotspots" for chemical transformations. For example, a carbonyl group (C=O) is known for its ability to participate in nucleophilic addition reactions, while a hydroxyl group (OH) often undergoes dehydration reactions. Understanding the structure of these functional groups allows chemists to predict and control the chemical behavior of molecules.
Furthermore, the spatial arrangement of atoms in a molecule, its stereochemistry, can dramatically impact its reactivity. Isomers, molecules with the same molecular formula but different arrangements of atoms, can exhibit vastly different chemical properties. For example, enantiomers, mirror images of each other, often display opposite biological activities. This understanding of stereochemistry is crucial in areas such as drug development, where subtle differences in structure can lead to significant changes in efficacy and safety.
Structure and Functionality: A Symphony of Properties
The structure of an organic molecule is directly linked to its physical and chemical properties. These properties determine how the molecule interacts with its environment, influencing its applications. For example, the presence of long, nonpolar hydrocarbon chains in a molecule can lead to its hydrophobic nature, making it suitable for use as a lubricant or solvent. Conversely, the presence of polar functional groups can render a molecule hydrophilic, allowing it to dissolve in water and participate in biological processes.
The structure of a molecule also dictates its physical state at room temperature. Linear molecules with weak intermolecular forces tend to be gases, while branched molecules with stronger intermolecular forces are more likely to be liquids or solids. Understanding these relationships between structure and properties is essential for designing molecules with specific functionalities for various applications.
The Importance of Structure: A Catalyst for Progress
The understanding and manipulation of molecular structure form the cornerstone of organic chemistry. This knowledge enables chemists to:
- Design new drugs and pharmaceuticals: By understanding the structure of target molecules within the body, chemists can design drugs that specifically bind to and modify their function, leading to therapeutic effects.
- Develop novel materials: The structure of polymers, plastics, and other materials can be tailored to create materials with specific properties, such as strength, flexibility, or conductivity, for various applications.
- Optimize chemical processes: Understanding the structure of reactants and products allows chemists to optimize reaction conditions and develop efficient synthetic routes for the production of valuable chemicals.
- Unravel the mysteries of nature: By studying the structures of natural products, chemists can uncover the secrets of biological systems and develop new technologies inspired by nature.
FAQs
Q: What is the difference between a molecule’s structure and its conformation?
A: A molecule’s structure refers to the arrangement of atoms within the molecule, including the types of bonds and their angles. Conformation, on the other hand, describes the spatial arrangement of atoms in a molecule that can change due to rotation around single bonds. While structure is fixed, conformation can be dynamic and influenced by factors like temperature and solvent.
Q: How can I determine the structure of an unknown organic molecule?
A: Several techniques are employed to determine the structure of unknown organic molecules. These include:
- Spectroscopy: Techniques like nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy provide information about the types of atoms and functional groups present in the molecule.
- Mass spectrometry: This technique determines the molecular weight and fragmentation pattern of the molecule, providing insights into its structure.
- X-ray crystallography: This technique uses X-rays to determine the precise three-dimensional arrangement of atoms in a crystalline solid.
Q: How does structure relate to the concept of chirality?
A: Chirality, or handedness, arises when a molecule cannot be superimposed on its mirror image. This is often due to the presence of a chiral center, typically a carbon atom bonded to four different substituents. The structure of a chiral molecule dictates its chirality, and this property can have significant implications for its biological activity and interactions with other molecules.
Tips for Studying Organic Chemistry
- Focus on understanding the basics: A strong foundation in atomic structure, bonding, and functional groups is crucial for success in organic chemistry.
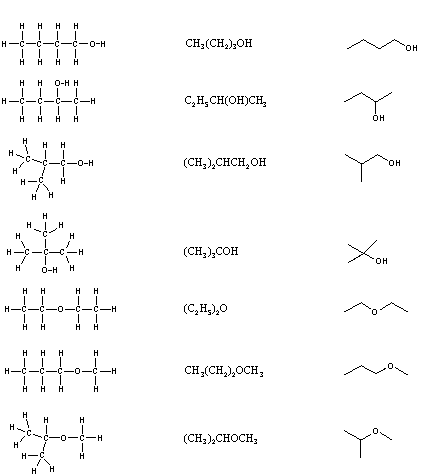
- Practice drawing structures: Drawing structures helps solidify your understanding of molecular geometry and allows you to visualize the relationships between structure and properties.
- Build models: Physical models of molecules can be helpful for visualizing three-dimensional structures and understanding the concept of conformation.
- Use flashcards: Flashcards can be a useful tool for memorizing the names, structures, and properties of common functional groups and reactions.
- Study in groups: Discussing concepts and solving problems together can enhance your understanding and retention.
Conclusion
The structure of organic molecules is a fundamental concept that underpins the entire field of organic chemistry. By understanding the relationships between structure, reactivity, and functionality, chemists can design new molecules with specific properties and applications. This knowledge is essential for advancing fields like medicine, materials science, and environmental chemistry, driving innovation and solving critical global challenges. The study of organic chemistry is a journey of discovery, where the exploration of molecular structure unlocks a universe of possibilities.




Closure
Thus, we hope this article has provided valuable insights into The Power of Structure: A Deep Dive into the World of Organic Chemistry. We hope you find this article informative and beneficial. See you in our next article!